Graph of the Day: Arctic Ozone Loss from 2010 to 2011
Caption by Mike Carlowicz and Kristyn Ecochard
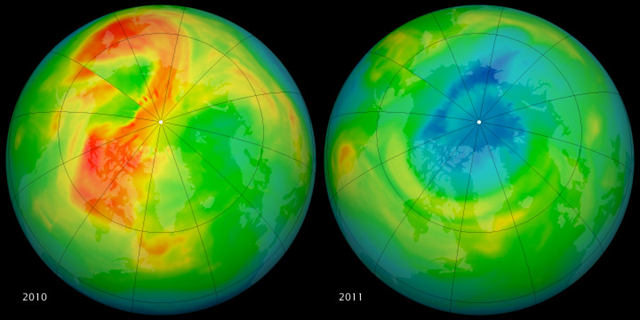
30 March 2011 Recent observations from satellites and ground stations suggest that atmospheric ozone levels for March in the Arctic were approaching the lowest levels in the modern instrumental era. What those readings mean for the remainder of the year is unclear. But what they mean for the long-term is that the recovery from human-induced ozone depletion is an uneven climb. These maps of ozone concentrations over the Arctic come from the Ozone Monitoring Instrument (OMI) on NASA’s Aura satellite. The left image shows March 19, 2010, and the right shows the same date in 2011. March 2010 had relatively high ozone, while March 2011 has low levels. The large animation file (linked below the images) shows the dynamics of the ozone layer from January 1 to March 23 in both years. In mid-March, scientists from Germany’s Alfred Wegener Institute announced that Arctic ozone levels had been cut in half in the waning weeks of winter, according to a network of 30 ozone sounding stations spread around the region. Data from OMI (shown above) also confirmed a depletion. OMI is a spectrometer that measures the amount of sunlight scattered by Earth’s atmosphere and surface, allowing scientists to assess how much ozone is present at various altitudes, particularly the stratosphere. Ozone is destroyed when chlorine- or bromine-based compounds—especially chlorofluorocarbons (CFCs) —break into their free atoms and combine with the oxygen. That process is amplified when the stratosphere is especially cold, which it has been in recent weeks. “This depletion is not necessarily a big surprise,” said Paul Newman, an atmospheric scientist and ozone expert at NASA’s Goddard Space Flight Center. “The ozone layer remains vulnerable to large depletions because total stratospheric chlorine levels are still high, in spite of the regulation of ozone-depleting substances by the Montreal Protocol. Chlorine levels are declining slowly because ozone-depleting substances have extremely long lifetimes.” The concentration of ozone in the Arctic atmosphere varies greatly from year-to-year, and ozone “holes” do not form consistently like they do in Antarctica. “Last winter, we had very high lower stratospheric temperatures and ozone levels were very high; this year is just the opposite,” Newman said. “The real question is: Why is this year so dynamically quiet and cold in the stratosphere? That’s a big question with no good answer.” Scientists will be watching in coming months for possible increases in the intensity of ultraviolet radiation (UV) in the Arctic and mid-latitudes, since ozone is Earth’s natural sunscreen. “We need to wait and see if this will actually happen,” Newman said. “It’s something to look at but it is not catastrophic.” On a global scale, the ozone layer is still on a long-term course for recovery. But for decades to come, there remains a risk of major ozone losses on yearly or regional scales.