Acid test: The world’s seas are becoming more acidic. How much that matters is not yet clear. But it might matter a lot.
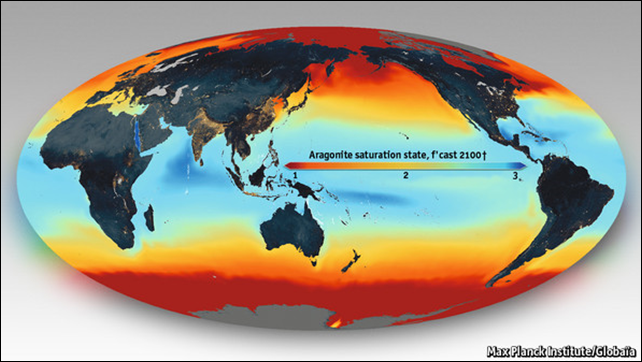
23 November 2013 (The Economist) – Humans, being a terrestrial species, are pleased to call their home “Earth”. A more honest name might be “Sea”, as more than seven-tenths of the planet’s surface is covered with salt water. Moreover, this water houses algae, bacteria (known as cyanobacteria) and plants that generate about half the oxygen in the atmosphere. And it also provides seafood—at least 15% of the protein eaten by 60% of the planet’s human population, an industry worth $218 billion a year. Its well-being is therefore of direct concern even to landlubbers. That well-being, some fear, is under threat from the increasing amount of carbon dioxide in the atmosphere, a consequence of industrialisation. This concern is separate from anything caused by the role of CO2 as a climate-changing greenhouse gas. It is a result of the fact that CO2, when dissolved in water, creates an acid. That matters, because many creatures which live in the ocean have shells or skeletons made of stuff that dissolves in acid. The more acidic the sea, the harder they have to work to keep their shells and skeletons intact. On the other hand, oceanic plants, cyanobacteria and algae, which use CO2 for photosynthesis, might rather like a world where more of that gas is dissolved in the water they live in—a gain, rather than a loss, to ocean productivity. Two reports attempting to summarise the world’s rather patchy knowledge about what is going on have recently been published. Both are the products of meetings held last year (the wheels grind slowly in environmental bureaucracy). One, in Monterey, California, looked at the science. The other, in Monaco, looked at possible economic consequences. Together, the documents suggest this is an issue that needs to be taken seriously, though worryingly little is known about it. […] The variable people most worry about is called omega. This is a number that describes how threatening acidification is to seashells and skeletons. Lots of these are made of calcium carbonate, which comes in two crystalline forms: calcite and aragonite. Many critters, especially reef-forming corals and free-swimming molluscs (and most molluscs are free-swimming as larvae), prefer aragonite for their shells and skeletons. Unfortunately, this is more sensitive to acidity than calcite is. An omega value for aragonite of one is the level of acidity where calcium carbonate dissolves out of the mineral as easily as it precipitates into it. In other words, the system is in equilibrium and shells made of aragonite will not tend to dissolve. Merely creeping above that value does not, however, get you out of the woods. Shell formation is an active process, and low omega values even above one make it hard. Corals, for example, require an omega value as high as three to grow their stony skeletons prolifically. As the map above shows, that could be a problem by 2100. Low omega values are spreading from the poles (whose colder waters dissolve carbon dioxide more easily) towards the tropics. The Monterey report suggests that the rate of erosion of reefs could outpace reef building by the middle of the century, and that all reef formation will cease by the end of it. Other species will suffer, too. A study published in Nature last year, for example, looked at the shells of planktonic snails called pteropods. In Antarctic waters, which already have an omega value of one, their shells were weak and badly formed when compared with those of similar species found in warmer, more northerly waters. Earlier work on other molluscs has come to similar conclusions. [more]
How much it matters? It's life or death for the world's marine life, or more specifically, death.
Acidic oceans will completely disrupt the marine food chain and even the oxygen produced on Earth as acidification destroys everything.
The oceans will die. The Earth will die. Oh, and humans will die too, but the religious idiots will all think that is a good thing.