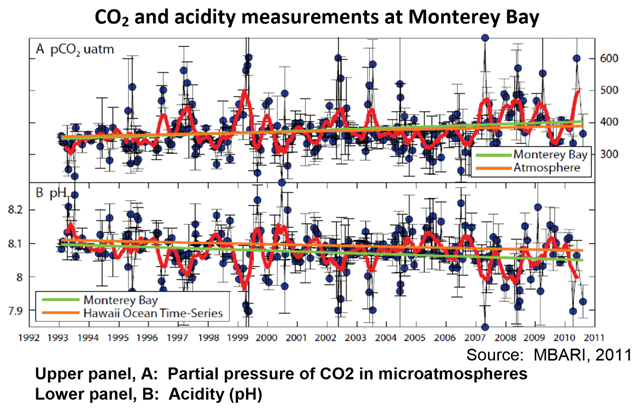
Graph of the Day: CO2 and acidity measurements at Monterey Bay, 1993-2010
8 August 2013 (CalEPA) – Carbon dioxide (CO2) is considered to be the largest and most important anthropogenic driver of climate change (see Atmospheric Greenhouse Gases indicator, page 19). CO2 is continuously exchanged between land, the atmosphere, and the ocean through physical, chemical, and biological processes (IPCC, 2007c). The ocean absorbs nearly one quarter of the CO2 released into the atmosphere by human activities every year. As atmospheric CO2 levels increase, so do CO2 levels in the ocean, changing the chemistry of seawater—a process called ocean acidification. Ocean acidification has been referred to as the “other CO2 problem” because it is, like climate change, the result of increasing levels of CO2 in the atmosphere (Doney, Fabry et al., 2009). The pH (a measure of acidity) of ocean surface waters has already decreased by about 0.1 unit since the beginning of the Industrial Revolution, from 8.2 to 8.1 (NOAA, 2010). Levels of CO2 in seawater are governed by both chemical and biologically-mediated reactions (photosynthesis, respiration, and calcium carbonate precipitation and dissolution). The air-sea exchange of CO2 is determined largely by the difference in the partial pressure of CO2 (pCO2) between the atmosphere and the ocean. When seawater pCO2 is lower than the atmospheric pCO2, seawater takes up CO2 from the overlying air; when it is greater than the atmospheric pCO2, CO2 is emitted to the air (Takahashi et al., 2010). CO2 in seawater reacts with water to form carbonic acid (H2CO3), most of which dissociates into a hydrogen ion (H+) and a bicarbonate ion (HCO3-); some of the H+ subsequently reacts with carbonate (CO3-2) to form more HCO3- ions. The net result of adding CO2 to seawater is an increase in H+—which increases seawater acidity and lowers seawater pH—and in HCO3-, along with a reduction in CO3-2 (IPCC, 2007c). H+ concentration (acidity) is measured on the pH scale (pH = -log[H+]), which is an inverse scale, so increases in H+ correspond to decreases in pH. Thus, the lower the pH, the more acidic the solution. It is notable that the increase in oceanic CO2 over the last two decades is consistent with the atmospheric increase. The rise in seawater pCO2 has been accompanied by declining pH (NOAA, 2010). In the presence of sunlight, phytoplankton in surface waters convert CO2 to organic matter through photosynthesis. A fraction of the organic matter sinks below the surface where it is decomposed, causing vertical variations in the concentrations of inorganic carbon species (CO2, HCO3-, and CO3-2) and pH. Another important biological process is the production of calcium carbonate by marine life (mostly in the form of the minerals calcite and aragonite) to serve as skeletons or hard protective structures (NAS, 2010). The vertical variation of pH in the ocean has been found to vary with geographic location, particularly as a function of latitude. pH also varies on time scales ranging from daily to inter-annual or longer, reflecting changes in biological processes that affect H+ concentration, as well as chemical and physical processes that may change the H+ content of a water mass or large-scale circulation patterns. For example, cyclical seasonal variations occur, as the intensity of biological processes varies with season, and the solubility of CO2 varies with temperature (NAS, 2010). Changes associated with large-scale climate oscillations such as El Niño and the Pacific Decadal Oscillation can alter the oceanic CO2 sink/source conditions through seawater temperature changes as well as through ecosystem variations that occur via complex physical-biological interactions (Chavez et al., 2007). In coastal waters, the processes affecting acidification are much more complex than in the open ocean and deep waters due to the influence of freshwater and atmospheric inputs, organic matter and algal nutrients inputs from land, and processes in the underlying sediment. Acidification can be mitigated or intensified by high rates of production or respiration, respectively, in coastal waters that may be fueled by inputs of nutrients or organic matter from land (NAS, 2010). Monterey Bay is perhaps the only place on the West Coast where two carbon parameters have been sampled regularly since 1993 (MBARI, 2011). These data support the hypothesis that acidification is occurring along the West Coast (NOAA, 2010). Levels of pCO2 at the sea surface (upper panel of graph, measured in microatmospheres or µatm) have increased, while exhibiting considerable variability. The increase in seawater pCO2 has been slightly higher than the increase in atmospheric pCO2. During the same time period, pH has decreased (bottom panel of graph). The decrease in pH (and hence the increase in acidity) in Monterey Bay waters has been greater than that in the open ocean near Hawaii (MBARI, 2011). While the gradual process of ocean acidification has long been recognized, the ecological implications of such chemical changes have only recently been examined (Doney et al., 2009; NAS, 2010; NOAA, 2010). The best-documented and mostly widely observed biological effect is decreased calcification rates in a wide range of shell- forming organisms, including plankton, mollusks, and corals. There is also evidence that the changes in ocean water chemistry—including secondary chemical reactions that alter various forms of trace elements and nutrients—affect a range of biological processes in marine organisms, including the fixation and respiration of CO2, regulation of internal pH, and uptake of nutrients for growth. The indirect effects of acidification, in tandem with changes in oxygen, temperature, and other factors that are predicted to change, are likely to impact nearly every species in marine food webs to some extent through predator-prey interactions, increased prevalence of invasive species, changes in pathogen distributions, and alterations of physical ecosystem structure. Species responses and degrees of sensitivity will vary considerably. Similarly, certain ecosystem types are expected to be more vulnerable than others. Along the West Coast, ocean acidification adds to the already naturally high levels of CO2 in upwelled waters. Upwelling is the wind-driven movement of deep, cool, and CO2- and nutrient-rich ocean water to the surface, replacing the warmer, usually nutrient-depleted surface water. Upwelled waters also characteristically have lower saturation states for the major carbonate minerals (aragonite and calcite) than surface waters, making it more difficult for species with calcium carbonate shells or skeletons to synthesize or maintain their shells. Many economically and ecologically important West Coast species are expected to show direct responses to acidification; bivalves, for example, are economically valuable, while also serving an ecological role in providing limiting substrate for other species. Recent observations indicate that bivalve shellfish hatcheries on both the West and East Coasts are experiencing a decline in production. Other stressors already impact coastal ecosystems—including fishing pressures, input of chemical contaminants, exotic and invasive species—and additional pressures are likely to occur as a result of the overarching effects of climate change (NOAA, 2010).
Indicators of Climate Change in California