Graph of the Day: Surface Ocean pCO2 and pH at the Atmospheric Mauna Loa Observatory, 1988–2008
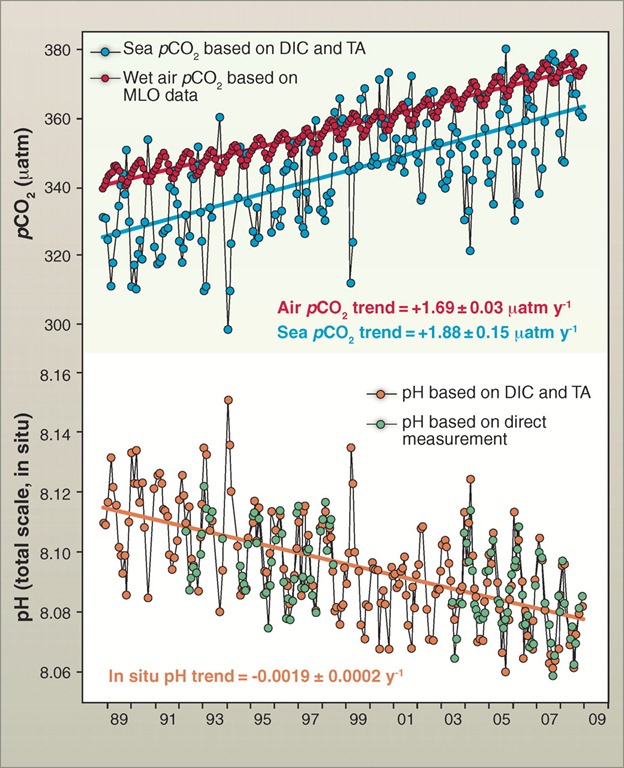
Time series of (top) atmospheric CO2 and surface ocean pCO2 and (bottom) surface ocean pH at the atmospheric Mauna Loa Observatory (MLO) on the island of Hawai‘i and Station ALOHA in the subtropical North Pacific north of Hawai‘i, 1988–2008. Doney, 2010, adapted from Dore et al, 2009. Ocean acidification is documented clearly from ocean time-series and survey measurements over the past two decades (Fig. 2) (26, 27). From preindustrial levels, contemporary surface ocean pH has dropped on average by about 0.1 pH units (a 26% increase in [H+]), and additional declines of 0.2 and 0.3 pH units will occur over the 21st century unless human CO2 emissions are curtailed substantially (28). Surface ocean CaCO3 saturation states are declining everywhere, and polar surface waters will become undersaturated for aragonite when atmospheric CO2 reaches 400 to 450 ppm for the Arctic and 550 to 600 ppm for the Antarctic (29). Subsurface waters will also be affected but more slowly, governed by ocean circulation, with the fastest rates in the main thermocline and high latitudes where cold surface waters sink into the ocean interior. Many coastal waters naturally have low pH, a factor amplified by acid rain (30) and nutrient eutrophication (see below). The rates of change in global ocean pH and Ω are unprecedented, a factor of 30 to 100 times faster than temporal changes in the recent geological past, and the perturbations will last many centuries to millennia. The geological record does contain past ocean acidification events, the most recent associated with the Paleocene-Eocene Thermal Maximum 55.8 million years ago. But these events may have occurred gradually enough and under different enough background conditions for ocean chemistry and biology that there is no good paleo-analog for the current situation (31).
The Growing Human Footprint on Coastal and Open-Ocean Biogeochemistry, Science 18 June 2010: Vol. 328. no. 5985, pp. 1512 – 1516 DOI: 10.1126/science.1185198