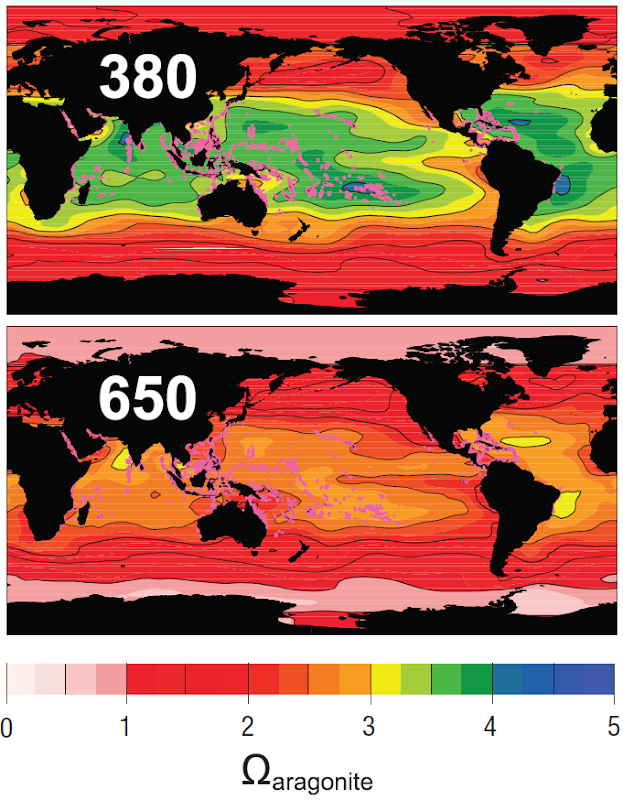
Graph of the Day: Aragonite Saturation and Ocean pH Change
Changes in surface ocean pH relative to pre-industrial values for different atmospheric CO2 stabilization levels, 380 ppm and 650 ppm plotted over existing shallow-water coral reef locations (shown as magenta dots). Results are obtained by adding model-predicted perturbations in geochemical fields to modern observations, except for the Arctic Ocean where results are model simulations only due to a lack of observations (Cao and Caldeira 2008). Further physical changes in the world’s oceans can be attributed to mounting concentrations of CO2 in the atmosphere. While increases in water temperature and fluctuations between fresh and saline water affect circulation at the surface and with vertical exchange, the repercussions from increasing concentrations of CO2 in the oceans introduce a separate but related threat. The ocean’s role in absorbing anthropogenic CO2 released into the atmosphere has been underway for over two centuries. This has altered the chemistry of the global ocean fundamentally, by acidifying the top 2,000 metre layer of the oceans’ waters and thus shrinking the total amount of ocean habitat where organisms that incorporate calcium carbonate (CaCO3) into their shells and skeletons can thrive (Caldeira and Wickett 2003, Sabine et al. 2004, Orr et al. 2005, Denman et al. 2007, Feely et al. 2008, Ilyina et al. 2009, Silverman et al. 2009).